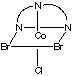
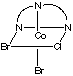
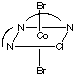
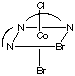
There are 4 geometric isomers with this formula. The dien ligand can coordinate either fac or mer, and there can be either a bromide or a chloride trans to the central nitrogen in the dien ligand.
 |
 |
|
|
|
 |
 |
|
|
|
This page is http://chemiris.labs.brocku.ca/~chemweb/courses/chem232/L7_SelfTest2_[Co(dien)Br2Cl].html
Created January 18, 2001 by M. F. Richardson
© Brock University, 2001